What Causes Thermal Runaway?
Editor's note: At a time when potentially risky energy storage technologies can be found in everything from consumer products to transportation and grid storage, UL Research Institutes helps to lay the groundwork for energy storage designs that are safe and reliable. As part of our work in this field, we want to share information on the foundations and current landscape of electrochemical safety.
The problem
One of the primary risks related to lithium-ion batteries is thermal runaway. Thermal runaway is a phenomenon in which the lithium-ion cell enters an uncontrollable, self-heating state. Thermal runaway can result in extremely high temperatures, violent cell venting, smoke and fire.
What causes thermal runaway?
Faults in a lithium-ion cell can result in a thermal runaway. These faults can be caused by internal failure or external conditions.
One example of such internal failure is an internal short circuit. In a lithium-ion cell, the cathode and anode electrodes are physically separated by a component called the separator. Defects in the cell that compromise the separator’s integrity can cause an internal short circuit condition that can result in thermal runaway. This is especially likely in cells of poor quality.
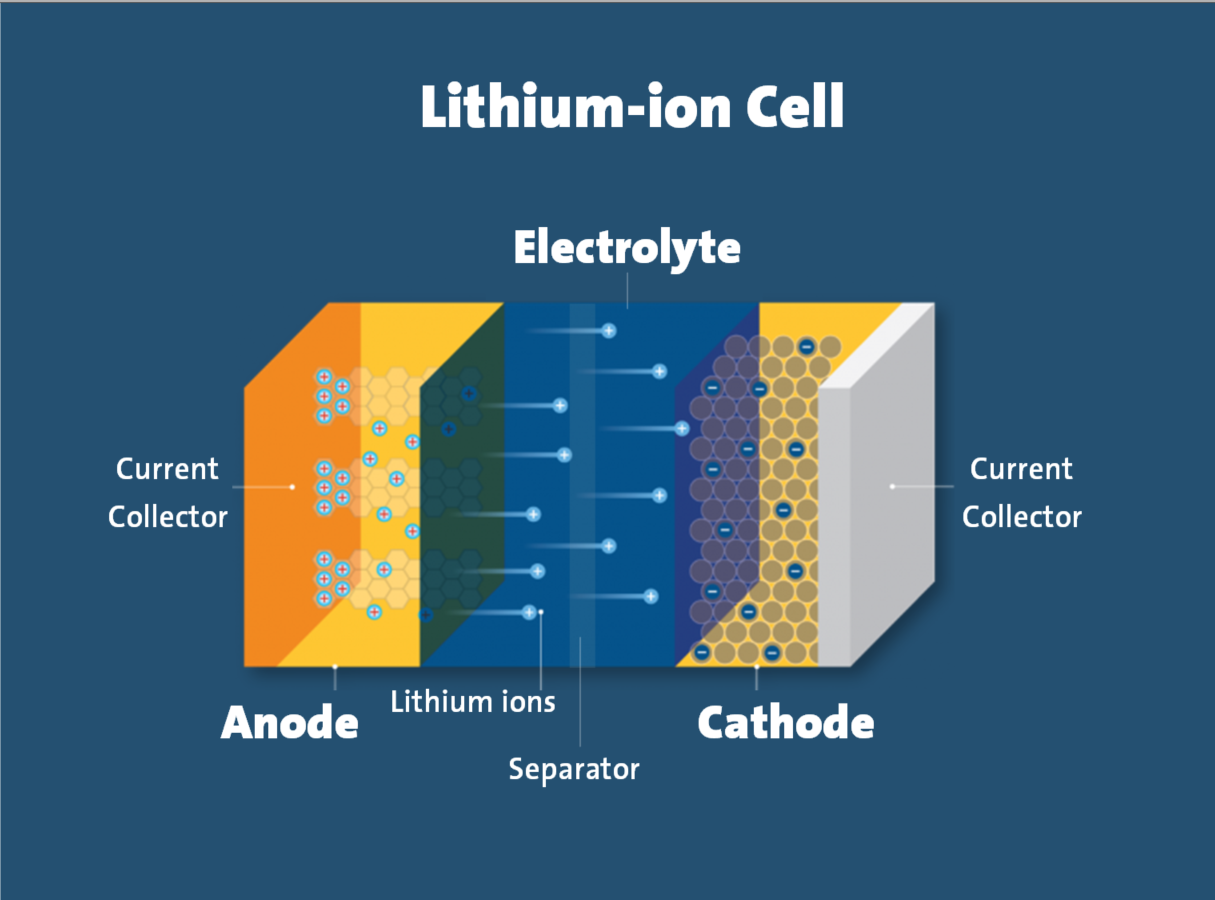
External, off-nominal conditions can also cause thermal runaway.
Examples of off-nominal conditions include:
- Overcharge: Can be due to incompatibility between cell and charger, or poorly designed battery management system (BMS)
- Multiple overdischarges followed by charge: Discharging the cell or battery below the cell manufacturer-recommended lower voltage threshold multiple times, then charging the cell
- External short circuit
- High- and low-temperature environments
Batteries are manufactured with controls intended to protect against off-nominal conditions. However, these conditions can lead to thermal runaway if the proper controls are not incorporated.